This post is for my friends and family who are not veterinarians.
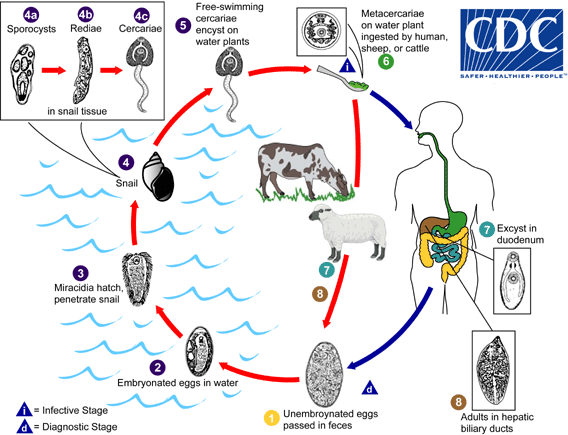
More recently, a 26- year old man living in California's Napa valley, was diagnosed with the condition "neurocysticercosis" (caused by the tapeworm Taenia solium), when he went to the emergency room complaining of a headache. Following his surgery and subsequent recovery, the man told the news channel this : “I just couldn’t believe something like that would happen to me. I didn’t know there was a parasite in my head trying to ruin my life.” (News story here). Whether the parasite was "trying to ruin" his life is a question that no one can answer. It was doing what it was wont to do, after all.The quick and brilliant diagnosis and treatment given to the man by the ER doctors saved the man's life.
Other recent stories have included the case of dwarf human tapeworm adults, Hymenolepis nana, in an immunocompromised human adult, that had showed malignant transformation (the worm had the tumor, that is). Here is the original research article in the New England Journal of Medicine (link here). And all at once, the "experts" who are neither veterinarians nor human physicians, have jumped on it, having a field day. The word "immunocompromised" seems to have been left out in news reports, and a whole host of blogposts have been written by these self professed "health writers", who have obviously done a lot of research on that veritable fount of wisdom and knowledge, called Google. The results speak for themselves, and the sole purpose appears to be fear mongering (like this one here).
In popular culture, neurocysticercosis has been featured in an episode of the popular show "House". Even comics and memes feature these dorso-ventrally flattened creatures (Comic here).

"Taenia saginata adult 5260 lores" by http://phil.cdc.gov/PHIL_Images/20031208/87d4bff74e41427cb278526bd9cbe76a/5260_lores.jpg. Licensed under Public Domain via Commons - https://commons.wikimedia.org/wiki/File:Taenia_saginata_adult_5260_lores.jpg#/media/File:Taenia_saginata_adult_5260_lores.jpg
As a Veterinarian and as a parasitologist, who does actual research in the field, I recommend a sane, balanced evaluation of tertiary literature, especially of blogposts and newspaper articles. In other words, don't believe everything written by self proclaimed "health experts", who are only arm-chair philosophers, who got their information from the first ten search results that Google (or worse Bing) brought up. [The letters that come after a person's name, gained after years of structured instruction at an academic institution, actually mean something. And if those letters are not MD, DVM, BVSc or something equivalent, it is best not to take parasitology advice from the people whose names precede the letters. (I assure you that we are not part of a conspiracy to get you)]
So, here are some things that you might be wondering about, which I had taken the liberty of answering, before you have voiced your questions.
*Q: Do humans get tapeworms?
A: Why, yes! They do. Both the tapeworms mentioned above (Taenia solium and Hymenolepis nana) are human tapeworms.
*Q: Are the two tapeworms mentioned above the only ones that humans get?
A: No, humans can be infected by other tapeworms too. If you ingest dog fleas that have the larval form of the dog tapeworm Dipylidium caninum, you can get infected by the adult of that species. If you eat uncooked fish with the larval stages of Diphylobothrium latum, you can be infected by adults of that species. If you drink water that have Cyclops or live microscopic floatsam that have the larval stages of Spirometra mansonoides , you can become the intermediate host for that tapeworm. If you ingest dog feces accidentally (I hope no one ingests dog feces intentionally), and if there were Taenia or Echinococcocus eggs in that feces, you can become the intermediate host for those tapeworms. There are other tapeworms that humans could potentially get, but notice how the words uncooked and feces keep recurring.
*Q: Practically speaking, did the man with the tapeworm cancer, actually have cancer?
A: No, the tapeworm had the cancer. Let me paraphrase that. The tapeworm did not cause a cancerous growth. It had the cancer itself. The man had HIV, and so his immune system could not do anything to prevent the spread of the cancerous tapeworm cells into his lymph nodes.
*Q: Are there many other humans who have tapeworm cancers that have been misidentified as human cancer?
A: Probably not. The case report in NEJM is the first of its kind reported. It began when pathologists who were looking at lymph node biopsies from the man realized that the neoplastic cells were smaller than human cells. Since pathologists are trained to look for such things, and since staging cancers involves these trained clinical pathologists to deliver their verdict on the malignancy of the cancer before the institution of treatment, you can be assured that they will be able to identify such things.
*Q: Can you/I get tapeworms that get cancers?
A: Not if you are a healthy adult. Generally, adult tapeworms cause very little effect on the their adult hosts. Unless you have hundreds of them, you will probably not even know that you have them.
The only way to get hundreds of them is to eat hundreds of the infective tapeworm larvae present in pork muscle. See the little white spots in the picture below? Those are tapeworm larvae. Know and recognize them.

(Source : http://www.austincc.edu/microbio/2704q/ts.htm)
Or in the case of Hymenolepis nana, eating the eggs shed by another human in his/her stools, who harbours the adult tapeworms in their intestines (who has not washed their hands after defecation).
*Q: If you have in the recent past eaten uncooked pork, how do you know if you are have tapeworms in your intestines?
A: Tapeworms live in the intestine and either shed eggs or shed their segments that burst open to release eggs. These get into the environment through the feces of the host. Feces can be examined under a microscope to see if you have tapeworms inside.
*Q: Are there treatments to get rid of adult tapeworms?
Yes. There is very effective medication approved for human use and for animal use, that can be prescribed to infected patients.
*Q: If prevention is better than cure, how can neurocysticercosis, and infection with adult tapeworms be prevented?
Here are some ways to help you avoid tapeworm infections:
1. Dispose human and pet animal waste properly.
2. Make sure that meat of all kinds is cooked properly. If you find that your beef or pork is "measly", do yourself a favor and throw it out. Make sure no one else eats it either.
Rare steaks can result in rare cases of tapeworms.
3. Alternately, freeze meat at -10C or less for more than 48 hrs.
4. Wash, wash, wash. Wash your hands before cooking, after cooking, before eating, after eating and especially after answering nature's call. Also, wash your hands after playing with your pets.
5. Take your pets to a vet for regular checks and make sure that including fecal examinations are done every time.